September 12, 2019 – Centerline Biomedical, Inc., has announced that it has been awarded its third small business grant from the NIH National Heart, Lung, and Blood Institute, with Cleveland Clinic as a subawardee. This Phase II grant, with a total budget of approximately USD $1.5 million, will support advancement of the company’s Intra-Operative Positioning System (IOPS) with augmented reality 3D guidance, navigation, and control (3D-GNC) for placing endovascular stents.
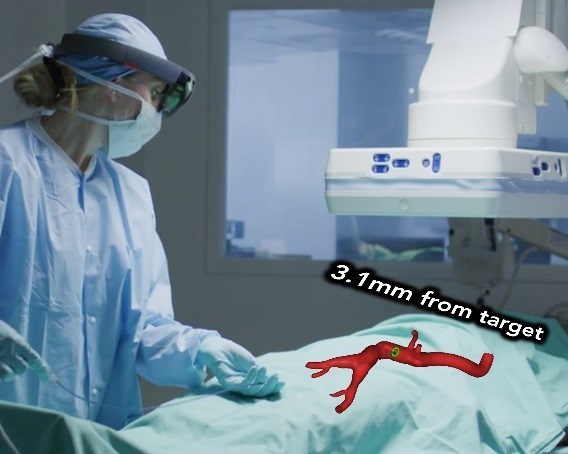
The company’s IOPS platform is a navigation system for minimally invasive surgery, leveraging anatomical mapping and electromagnetic tracking to provide three-dimensional color visualization and guidance in real time during endovascular interventions without the continuous use of X-ray radiation typical of the current standard of care. The first product, recently granted FDA 510(k) clearance, allows doctors to navigate catheters and guidewires through complex anatomy with intuitive and safe imaging. The pipeline technologies to be advanced and tested in this funded project will help bring the technology forward, with augmented reality visualization and software assistance in actual guidance.
IOPS with 3D-GNC represents a step forward when compared with X-ray fluoroscopy and provides a way to leverage the maturing capabilities of augmented reality and artificial intelligence. AR allows a doctor to see directly inside the body while the system provides real time information to support precise positioning. As the IOPS data platform grows the AI will be able to provide even more navigational aids based on prior surgical experience. Centerline stated that by simplifying complex procedures and making them faster, safer, and more accurate, it hopes to decrease costs while improving access to care.
“Adding this piece to our technology will have a multiplicative effect on the value to the clinician,” remarked Centerline Chief Technology Officer and Founder Vikash Goel. “We believe it will improve patient outcomes right away, and the strategic opportunities it opens for Centerline will make the IOPS technology much more impactful.”
This project builds upon the success of the Phase I grant, in which it was shown in a preclinical study that 3D-GNC was able to simultaneously reduce radiation dose while improving accuracy and shortening procedure times. This Phase II work will culminate in a first-in-human study and pave the way to realizing the full clinical and economic benefits the technology has to offer, according to the company.
Image credit: Centerline Biomedical, Inc.
About the author
Sam is the Founder and Managing Editor of Auganix. With a background in research and report writing, he has been covering XR industry news for the past seven years.

 In
In